Wholesale Rapid Diagnostic Test Kits Manufacturer
Fast Results
Most results are available within 3–10 minutes
High Accuracy
Over 99% sensitivity and specificity
Multiple Sample Types
More flexible and convenient detection
Easy to Operate
Convenient testing, no training required
Hongmian Wholesale Rapid Test Kits
Drug of Abuse Test Kit Wholesale
Bulk Multi-Drug Detection Kit for Opiates Amphetamine Hongmian POCT Manufacturer
Hongmian 6-Panel Rapid Drug Test High Quality Substance Abuse Dection Wholesale

OEM Quick Workplace Drug Testing Dip Cards Multi-Dip Substance Abuse Pathological Analysis Equipments

Multi-Panel Rapid Test Cup for Workplace Clinical Forensic Use Drugs Abuse Test Kits

Hongmian10 Panel Rapid Drug Test Set CE Approved Manual Urinary Substance Abuse Pregnancy Test Custom

High Quality 14 Panel Rapid Drug Test CE Approved Substance Abuse Pregnancy Test Kits Supplier
Infectious Disease Test Kit Wholesale

2-in-1 Rotavirus & Adenovirus Antigen Detection Kit Wholesale
HIV 12 & Syphilis 2-in-1 Detection Kit, Hongmian In Vitro Diagnostic Reagent Manufacturer
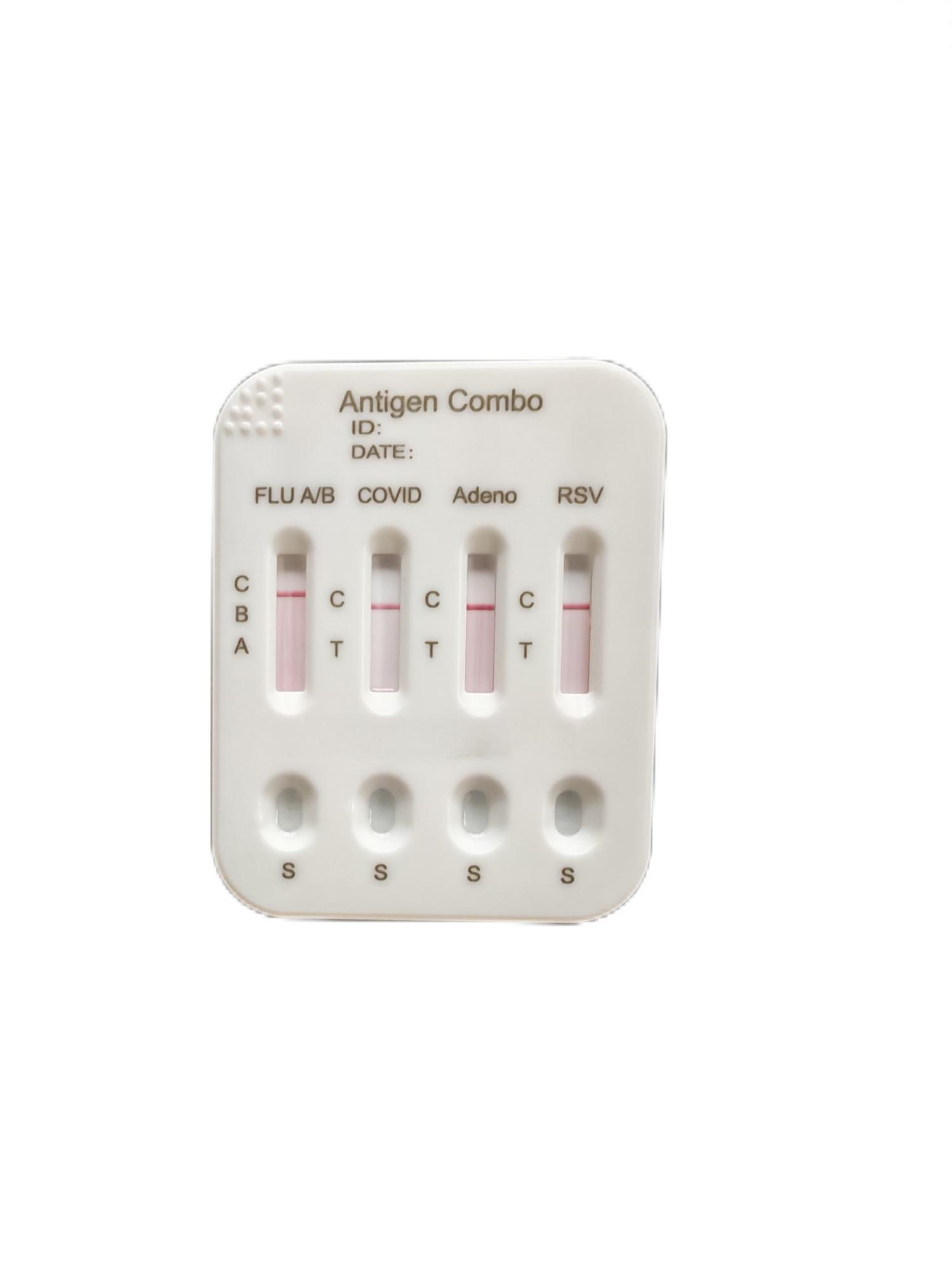
5-in-1 Detection Kit of COVID-19 Influenza A Influenza B RSV Adenovirus Antigens Custom

9-in-1 Detection Kit of COVID-19 Influenza A/Influenza B RSV MP Adenovirus Parainfluenza 1/2/3 Human Lung Disease Antigens Wholesale
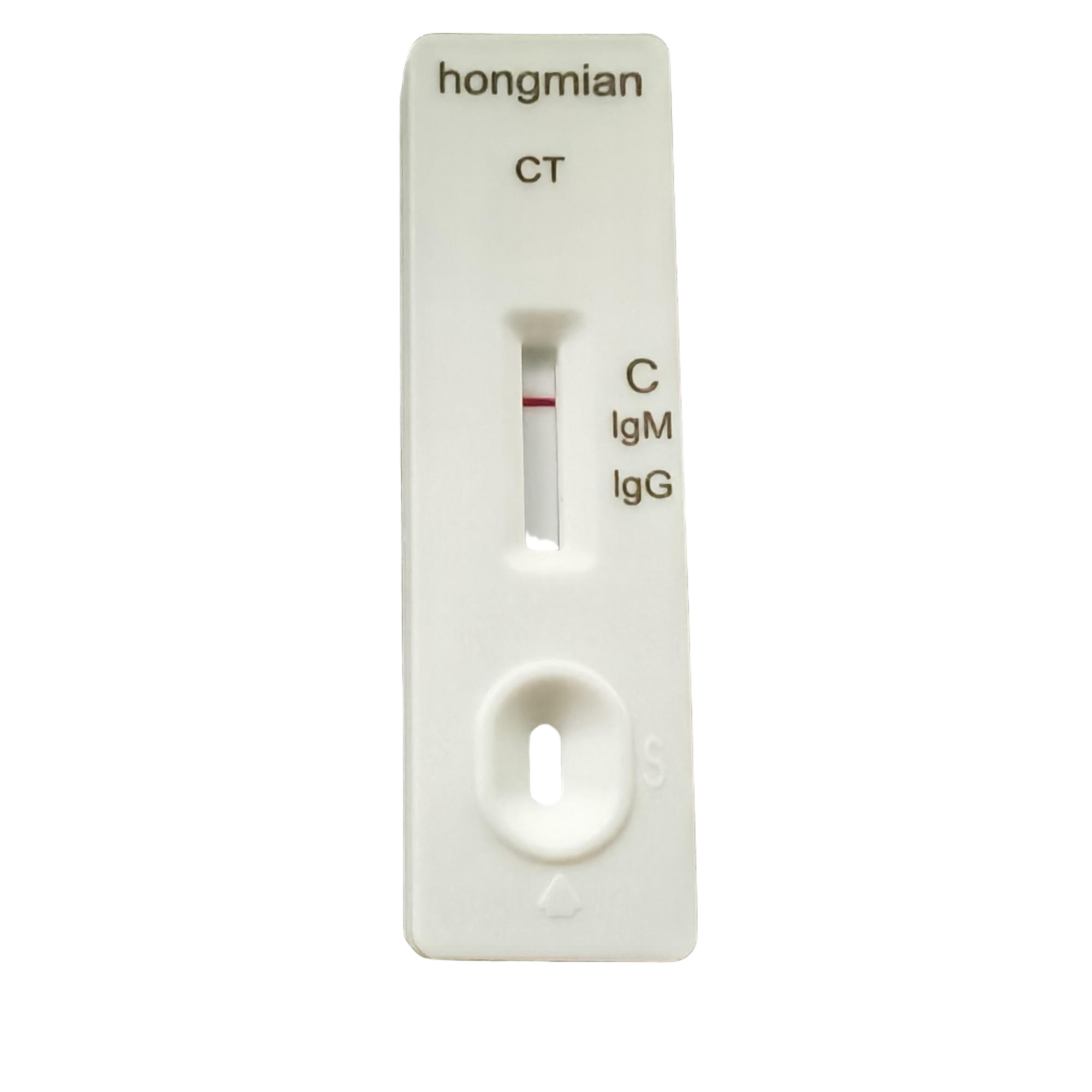
Chlamydia Trachomatis IgM IgG Rapid Test Kit Manufacturer
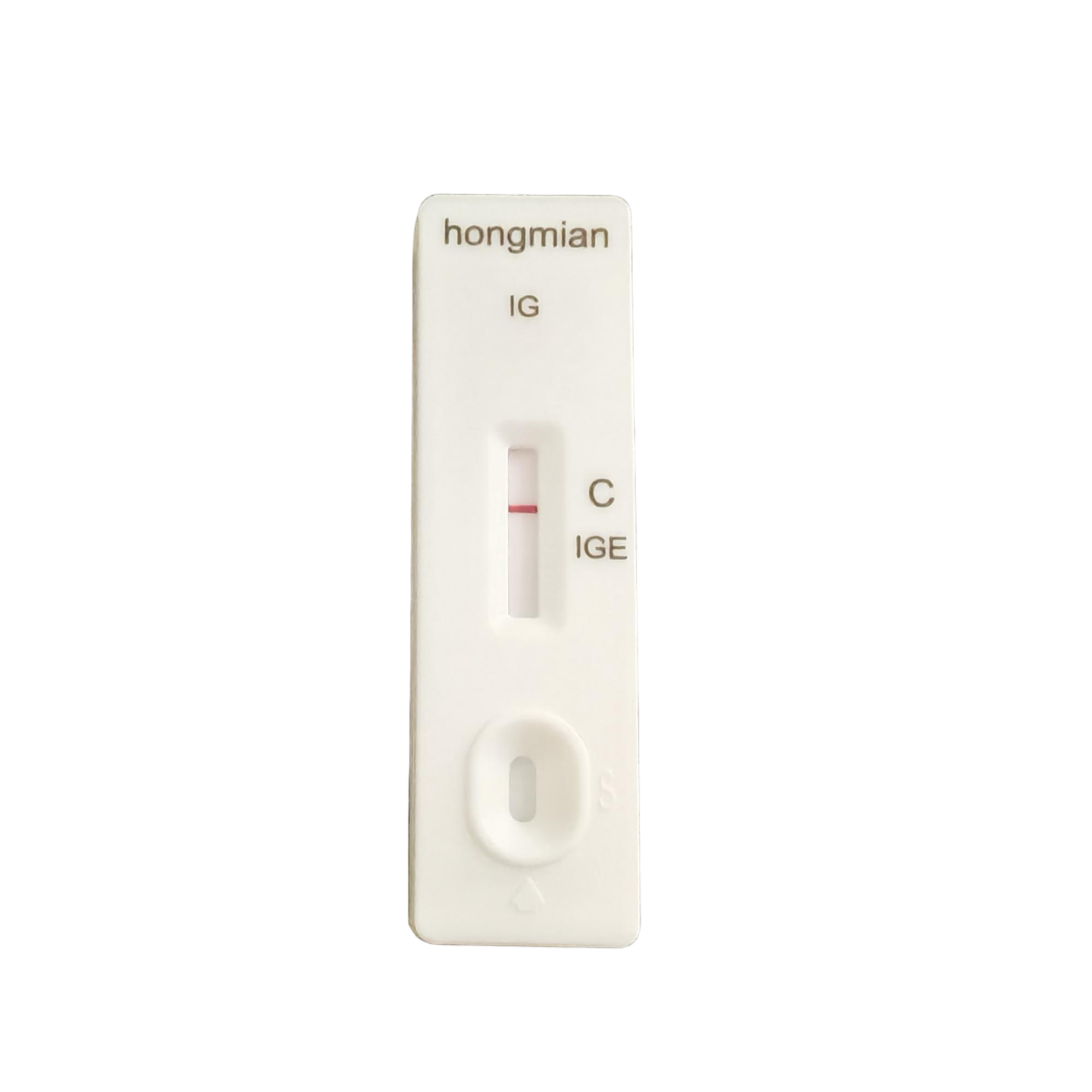
Immunoglobulin IGE Detection Kit, Hongmian Rapid Test Kits Supplier
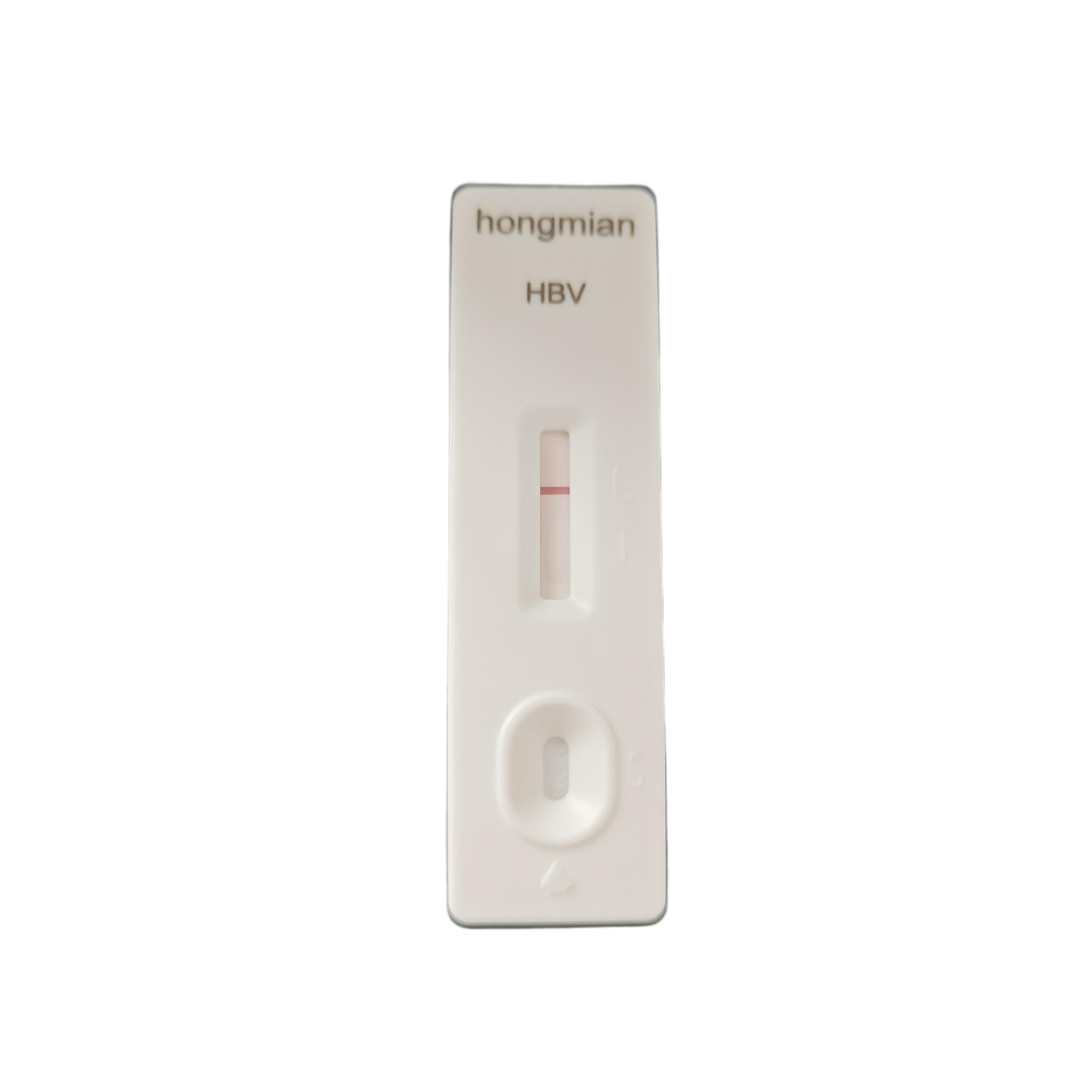
Hepatitis B Virus (HBV) Surface Antigen Rapid Test Kit POCT Manufacturer
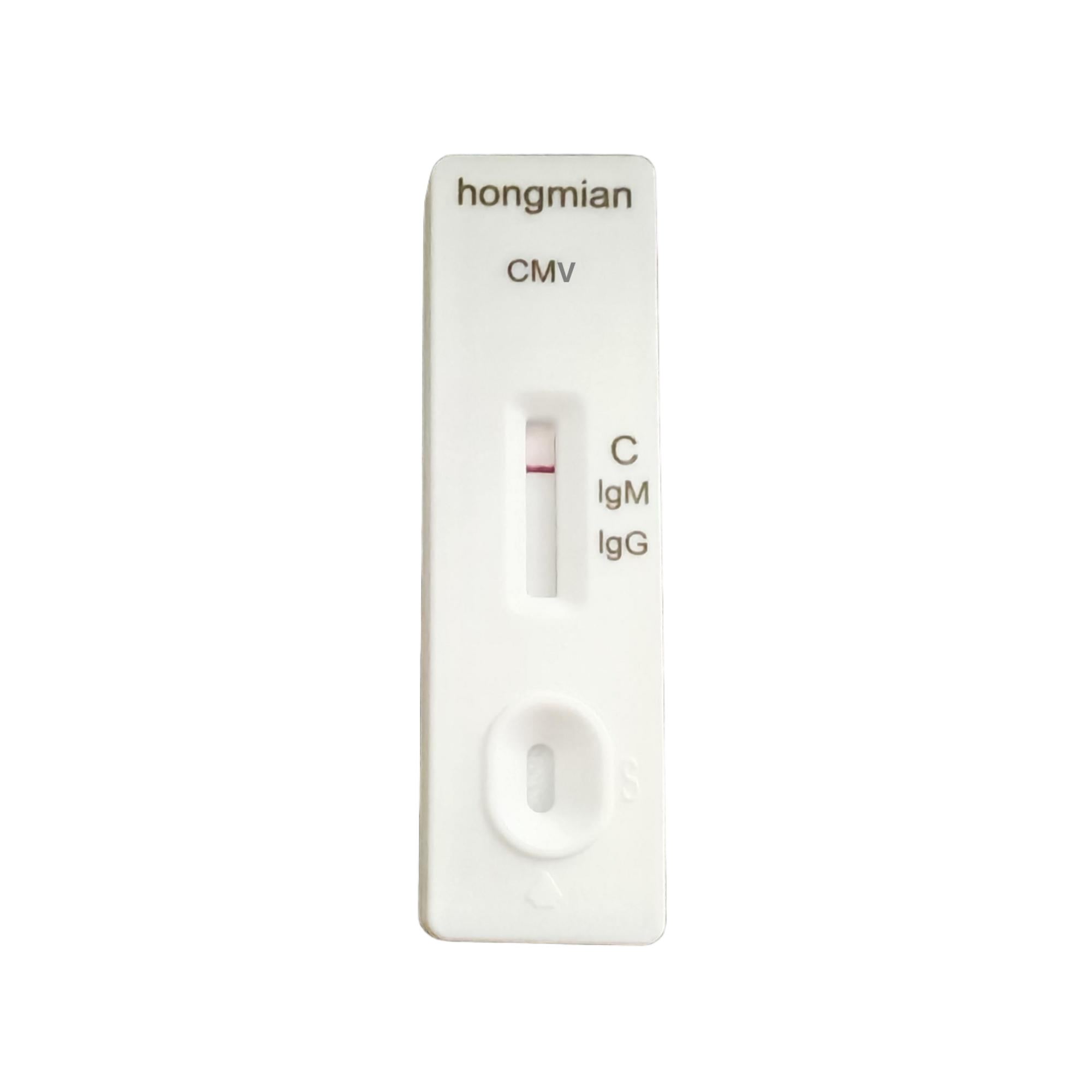
Cytomegalovirus IgM/IgG Antibody Detection Kit, Hongmian Rapid Test Kits Supplier
Cardiac Marker Test Kit Wholesale

4-in-1 Cardiac Marker Test Kit – Troponin I, CK, D-dimer & Isoenzymes
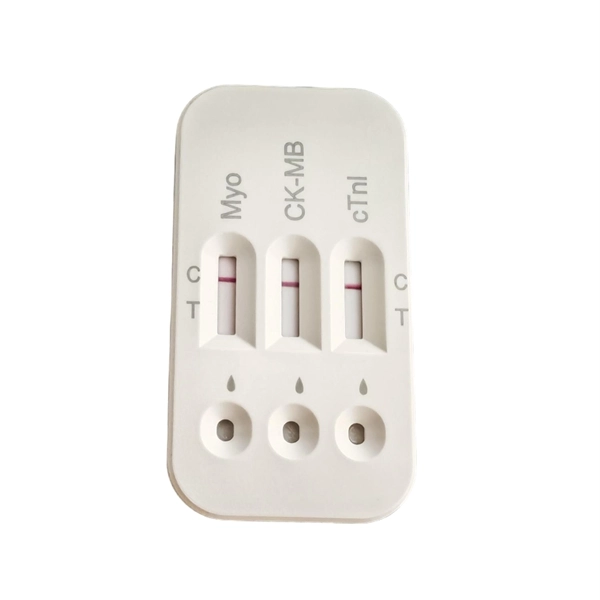
Cardiac Troponin I Myoglobin CK-MB Combined Test Kit for Pathological Analysis Bulk Supply
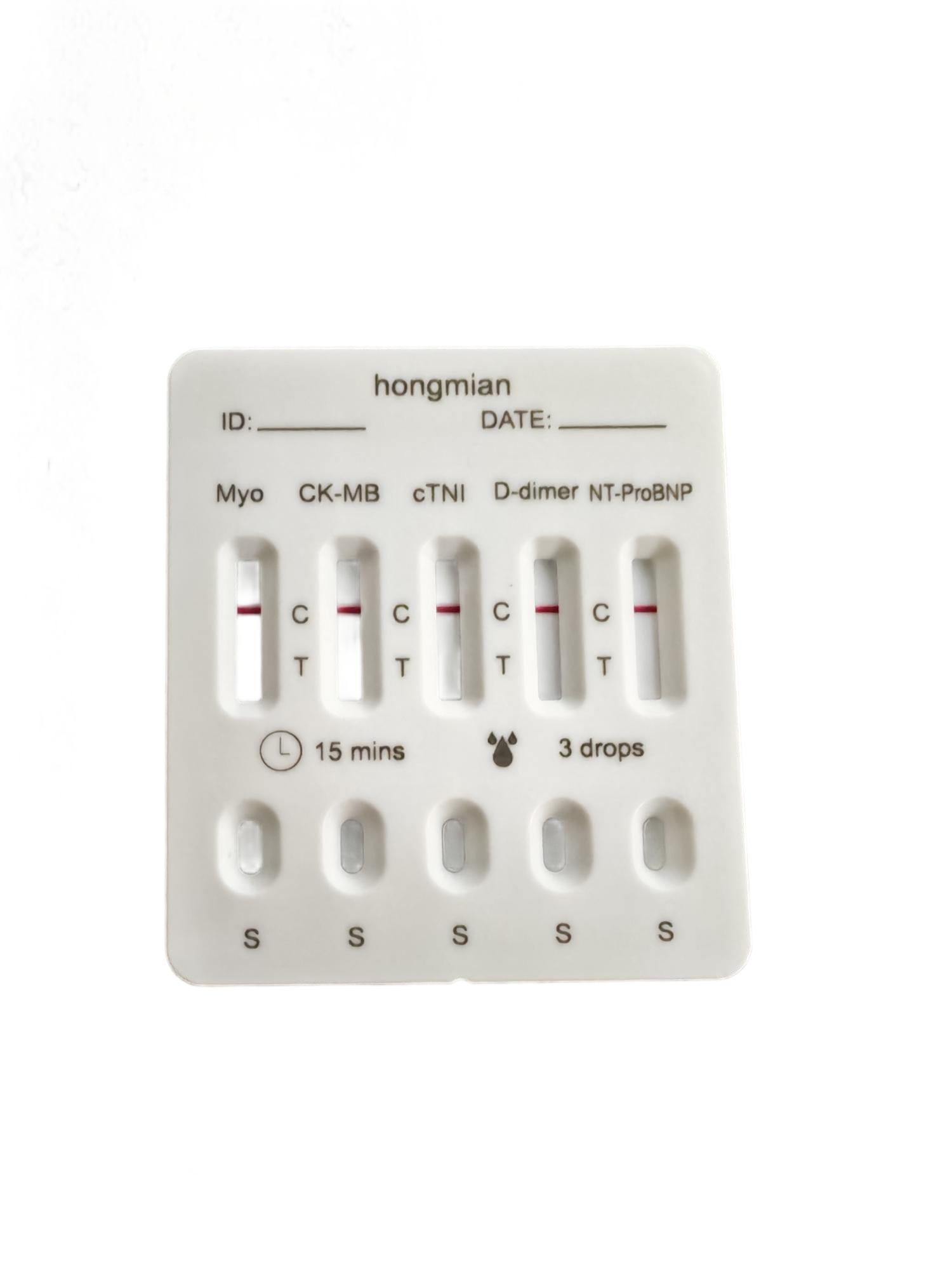
5-in-1 Cardiac Maker Test Kits Troponin I, CK, Isoenzyme & NT-proBNP Wholesale
Tumor Marker Test Kit Wholesale
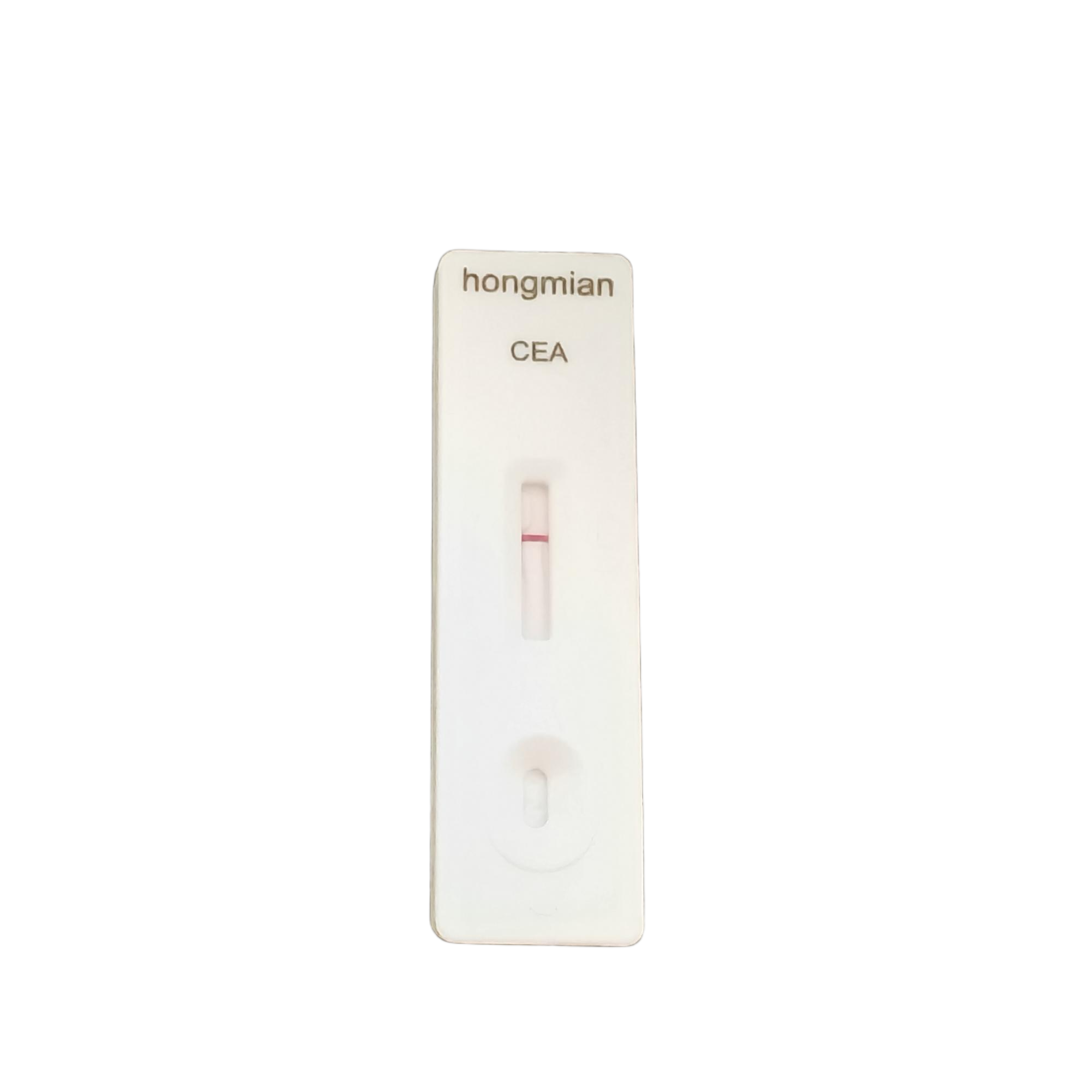
Carcinoembryonic Antigen Detection Kit CEA Rapid Test Kit for Tumor Maker Monitoring
Faecal Occult Blood Test for Human Occult Blood in Feces Detection FOB Rapid Test Bulk Supply
Transferrin Detection Kit TRF Rapid Test Kit for Early Screening for Colorectal Cancer POCT Wholesale
Prostate Specific Antigen (PSA)Rapid Test for Healthcare Management Supply
Fertility Test Kit Wholesale
Vitamin D Test Kit Wholesale
Custom Rapid Test Kits Solutions
Hongmian Wholesale and Custom Rapid Test Kits FAQs
Q1: What is the minimum order quantity (MOQ) with you?
A1: Our MOQ for our rapid test kits is 500 boxes/kits.
Q2: How much time does it take to get the sample?
A2: It will take around 10 days to prepare a sample, after confirming the instruction manual, the packaging, and the logo.
Q3: What are your payment terms and shipping time?
A3: We usually take T/T 50% advance payment, balance prior to shipment. Normal delivery time is 20–30 days based on order quantity and customization.
Q4: Is there a free sample?
A4: We could provide a quick test kit sample; they are charged.
Q5: Do you offer custom services?
A5: Yes. We provide OEM/ODM customization, including logo printing, packaging design, multi-language package inserts, and bundled test kits for multiple viruses.
Q6: Do you have stock?
A6: We hold regular stock for regular products in order to be delivered on time. Special custom products will be produced according to your requirements.
Q7: Which countries and regions do you export to?
A7: We can ship to many countries and regions worldwide, such as Africa, the Middle East, and Asia.
Q8: Are the key raw materials produced internally?
A8: Yes. We produce essential raw materials, including antibodies, antigens, and enzymes, to maintain a stable supply and consistent product performance.
Q9: Do you provide after-sales technical support?
A9: Yes. We are providing wide technical and customer support, from product information to operating information and regulatory guidance, in a manner that facilitates harmonious collaboration.